pH-stat and alpha-stat

I don't want to read this whole post, so just give me a really brief overview
What do you do if the patient is really cold and you want to do a blood gas analysis of their acid base balance?
Alpha-Stat Method:
You accept that pH and PaCO₂ will naturally change with changes in body temperature.
- As body temperature drops, pH naturally rises and PaCO₂ decreases
- You are not trying to "correct" these changes because they are a normal part of how the body adapts to cooling
- The blood gas sample is warmed to 37°C by the machine
- You interpret the results as if the patient were at 37°C, even if they are actually colder
pH-Stat Method:
You aim to keep pH at 7.4 and PaCO₂ at 5.3 kPa, even when the patient’s temperature changes.
- You actively intervene to normalise these values as if the patient were at 37°C, regardless of their actual body temperature
- The blood gas is measured at the patient’s actual temperature, and not warmed to 37°C
- The results are interpreted in the context of what the reference values would be at 37°C, but adjustments (like adding CO₂) are made to bring the patient's pH back to 7.40
Most people agree to just use Alpha stat and be done with the whole argument.
Let's start with Neutral
I'll clarify that we're not talking about 1940's Switzerland, a manual gearbox or facial expressions.
We're talking about water.
A pH of 7.0 is not always neutral.
Huh?
Correct. Remember that pH just relates to the concentration of H+.
We learn that pH 7.0 = neutral, but this isn't strictly true, neutral is actually when [OH-] = [H+].
This just happens to occur at pH 7.0 at room temperature.
If we have a very high concentration of H+, but an equally high concentration of OH-, then the pH is very low, but it's still 'neutral'.
This is our starting point definition, and it's important, because the pH at which 'neutral' happens actually changes with temperature.
Why pH of fluid changes with temperature
- Water (H2O) splits into (OH-) and (H+) as a natural and reversible equation
- At 25°C for pure water this ends up giving a pH of 7.0, which we call neutral and use as our reference range
As temperature drops
- Dissociation or ionisation is an endothermic process
- At lower temperatures, molecules move more slowly, and there's less splitting (ionisation) into H+ and OH-, meaning the pH is higher and the water is measured as more alkalotic
- It is still neutral, however, as there are equal numbers of H+ and OH-
- The reverse happens with an increase in temperature
- For each 1 degree change in temperature, the pH changes by 0.017
This change with temperature is called the ionisation constant of water (Kw)
- At body temperature, water has a pH of 6.8
- At 0°C, the pH is around 7.5
- At -35°C the pH is 8.5
Thus the 'neutral', normal operating pH inside each of a healthy human's cells is 6.8.
Hence a 'normal' or 'neutral' pH for warm human blood is actually around 7.4.
Let's heat things up
Now, what's really cool is that somehow, intracellular pH manages to stay at 'neutral' at a whole range of temperatures.
- This means there must be a buffer at play
- One of these buffers is the imidazole histidine residue found in proteins
The imidazole residue can accept or release a proton, and the pKa of this process is around 6.8, making it perfect as an intracellular buffer (A buffer is most effective when operating at a pH close to it's own pKa).
What's even cooler, is this pKa changes with temperature, to match the pH of the intracellular environment, so it'll just keep on buffering, and keeping the balance of OH- and H+ ions equal - which is the definition of neutral.
Noice.
Why is this my problem?
If you test a sample of blood to see what the measured pH is, you have two options.
- You can test it at whatever temperature it happens to be
- Or you can warm it to some pre-determined number before analysing it
You will therefore get two different numbers depending on what method you choose, because the pH of the blood will change with temperature.
Simple enough so far.
What about CO2?
A cold patient will have a low PaCO2 for two reasons:
- Their metabolic rate is usually lower, so they produce less
- Their blood is better at dissolving CO2, so there is less available to exert a partial pressure
The basic idea
- Cold fluid will dissolve more gas, because the dissolving process is exothermic
- This is why rising sea temperatures is bad news, as it releases yet more dissolved CO2 into the atmosphere
- If the solubility is higher then there is less partial pressure exerted by that gas (Henry's law)
- If you take blood from a cold patient, and warm it up, more CO2 will be released, the partial pressure will increase, and the reading you get on the machine will be higher.
The PaCO2 reading on a blood gas will be higher than the actual PaCO2 in a cold patient.
- We've already shown how pH increases with temperature
- Cold blood samples will therefore appear alkalotic and hypocarbic
So, you can either measure it cold, or warm it up - how do you choose?
Your two options
Option 1: Alpha stat
"Let the pH change naturally with temperature because the body adjusts to it, and that's what is supposed to happen."
- You take any sample that enters the machine and warm it to 37°C
- You measure the newly-warmed sample and use your normal (37°C) reference ranges when interpreting the results
- You accept that the measured numbers will be slightly different to the actual values in the cold patient's blood, because they will have changed during the warming process
- You accept that nature's pretty slick at figuring this stuff out and just treat them like normal
Keep it simple - heat the sample to normal body temperature, and then interpret the numbers as if the patient has a normal temperature.
Scroll to the bottom of the post if you want to know why it's called 'alpha'.
Option 2: pH stat
"Force the pH to stay the same, even if the temperature changes."
- Analyse the sample at the temperature at which it happens to be
- Take the numbers and compare them to your normal reference ranges
- Make deliberate changes to make the numbers look normal, because you don't trust nature and its devious schemes
A pHstat is a piece of chemistry laboratory equipment that ensures a solution stays at a set pH throughout the course of an experiment.
Kind of like your kidneys and lungs.
A cold sample will appear alkalotic and have a low CO2 (if you haven't warmed it up) compared to a warmed sample.
This will then tempt you to increase the CO2 by hypoventilating the patient.
- This will increase the PaCO2
- This will help push the oxyhaemoglobin curve to the right (remember the curve shifts to the left in hypothermia)
- It will also cause vasodilatation of cerebral blood vessels
- Together these two processes give better oxygen delivery to the brain
At least that's the theory.
Give me an example
You have a patient on bypass having cardiac surgery, and their core temperature is currently 20°C.
You take a blood gas sample and don't warm it up (pHstat):
- pH = 7.65
- PCO2 = 2.3 kPa
Then you take another sample and warm it to 37°C (alphastat):
- pH = 7.4
- PCO2 = 5.3 kPa
Your next steps in managing this patient are clearly going to be different depending on which technique you've used.
Let's talk O2
As temperature drops, the solubility of all gases increases, so the partial pressure exerted by these gases drops.
For this reason, a cold patient's PaO2 will also be lower than normal, even though there's actually more oxygen dissolved in the blood - the overall content is higher.
The overall oxygen content of the blood increases for two reasons:
- increased solubility in the plasma
- huge left shift of the oxyhaemoglobin curve
This massively increases the affinity of haemoglobin for oxygen (by over 20x) and the overall number of oxygen molecules in the blood goes up.
This does not mean better oxygen supply to the tissues, remember, because the oxygen doesn't want to leave the blood or haemoglobin.
If you warm the sample up, and read the PaO2, the newly-warmed haemoglobin shifts it's curve to the right, and the plasma's ability to dissolve oxygen drops, and lots gets released, artificially increasing the PaO2, and giving you a falsely high reading.
The overall maths works out at a pleasingly reassuring answer:
- Clinically meaningful oxygen content doesn't change significantly with temperature, so there's no point in adjusting for temperature
Just tell me the answer
Most cardiac anaesthetists (who are dealing with deliberately chilly patients) generally use alpha stat because:
- It doesn't really make sense to artificially adjust the pH back to 7.4, because the pH of 'neutral' changes with temperature, and this is what the body is used to
- The body probably knows what it's doing after all these years of evolution
- If you start messing around correcting CO2 values at temperatures for which we have no robust reference ranges, you could make things a lot worse
I'm really keen to know about the Arrhenius and Brønsted-Lowry theories
Welcome, friend, we're so proud of you for joining us.
These are two slightly different definitions of what an acid actually is.
Arrhenius theory
- An acid is anything that increases the number of H+ ions
- A base is something that increases the number of OH- ions
The Arrhenius theory only applies to water-based solutions, and doesn't explain why ammonia acts like a base, because it doesn't actually increase the number of OH- ions.
Brønsted-Lowry theory
- An acid donates a proton
- A base accepts a proton
This theory can apply to all sorts of fluids, and also explains why ammonia acts like a base.
BUT the Brønsted-Lowry theory has no convenient definition of neutral.
What is the 'alpha' of imidazole residues?
We mentioned earlier that the protein imidazole residues change their operating pKa depending on temperature.
The alpha of the imidazole residues is the ratio of residues that are currently holding onto a proton
- It is the ratio of how many imidazole residues are protonated
- Because the pKa changes with temperature, the alpha remains stable even as temperature changes
- At any body temperature, around half of the residues are protonated (alpha = 0.55)
You do not need to know any of this, except that this is why it's called the 'alpha-stat' method.
Tell me about the frogs
Frogs are cool because they don't really control their own body temperature, but somehow still function in a variety of climates.
- At 5°C, their measured pH is above 8 and their PaCO2 is around 0.6 kPa
The cells don't care what the measured pH is, they just care about being neutral.
While they are cold-blooded and not exactly the perfect model for human physiology, it does demonstrate that pH and CO2 in living tissue can quite happily alter with temperature without compromising the wellbeing of the organism, and thus lends credence to the alpha stat approach of - just trust nature.
References and Further Reading
Useful tweets and resources
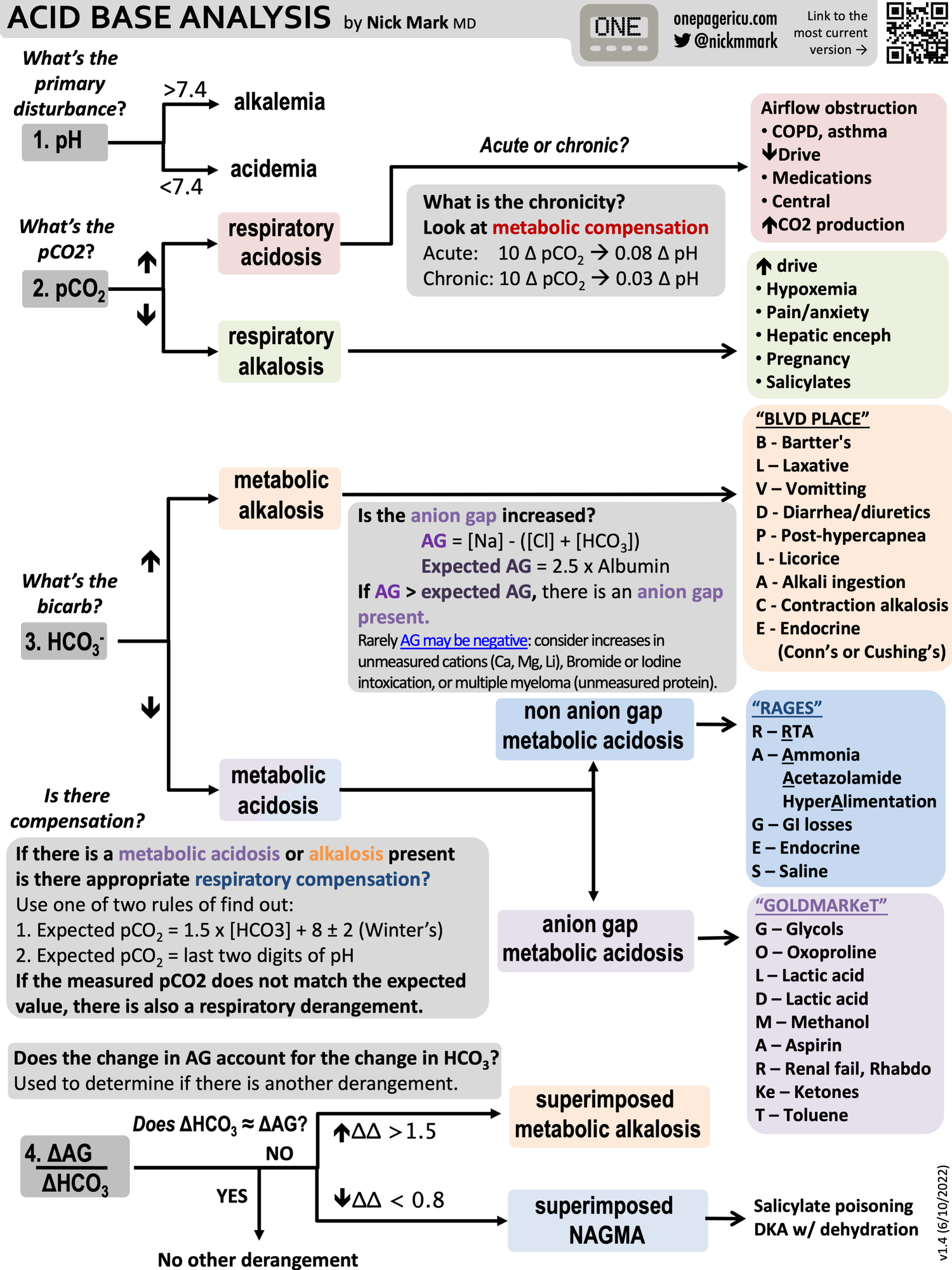
Check out this amazing flow chart from OnePagerICU (shared with permission)
By far the lowest pH I’ve ever seen in a patient awake and talking to me. Bicarbonate less than 5. Creatinine 1.4, glucose 217, ETOH 81 BHB 1.5 salicylate <2.5. Everyone else have the same thoughts as I have? pic.twitter.com/yoZmjzkSE2
— Loice Swisher (@L_swish) April 30, 2020
Continuing our #NewPeopleInTheICU series or #OnePagers:
— ICU OnePager (@OnePagerICU) June 10, 2022
5️⃣Interpreting Acid/Base - How do you tell the difference between a metabolic & respiratory #acidosis? What causes an anion gap acidosis (GOLDMARKeT), a non-anion gap acidosis (RAGES), or a metabolic #alkalosis (BLVD PLACE) pic.twitter.com/hfBlKS1RYv

