Malignant Hyperthermia

Take home messages
- Malignant Hyperthermia is vanishingly rare but exceedingly important to spot and manage early
- The clue is unexplained increasing CO2 and tachycardia
- Dantrolene takes ages to draw up and administer - so delegate to your team
- Learn how to prepare a workstation for a patient known to be MH susceptible
Your worst nightmare?
Ask an anaesthetist what they're most afraid of happening during an anaesthetic, and chances are they'll at least mention malignant hyperthermia.
It's terrifiying.
Imagine putting a car in neutral, switching off the rev limiter, and then standing on the accelerator. The engine gets faster, and faster, and hotter, and hotter and eventually can't take the strain and seizes up or breaks down entirely.
It's the human version of that.
It's a brutal combination of life threatening, difficult to spot quickly and ridiculously rare that makes it one of the anaesthetist's most fearsome nemeses.
But don't stress.
If you know what you're looking for, and what to do if you suspect it, you can fix it if you're quick - so read on.
A spot of history
The year is 1960, and the patient is a 21 year old student in Melbourne, who has just been hit by a car on the way to class and suffered a complex open tibial and fibular fracture.
His mother accompanied him to the emergency department whereupon their main concern was the fact that 10 close relatives had all died as a result of general anaesthesia.
They understandably weren't too keen on the idea of an operation.
Dr James Villiers was the anaesthetist, and upon taking what must have been rather a chilling pre-operative history, decided that ether must have been the problem, as it had been used for every single one of those fatal cases.
So he chose to use halothane instead.
Ten minutes after induction, the boy became hypotensive, tachycardic, cyanosed and exceedingly warm to the touch.
"The soda lime quickly expired and was changed".
Mercifully the reduction was quick, and the halothane switched off. The cause of the problem was thought to be blood loss, and luckily a neighbouring theatre was performing a cardiothoracic case, with ice available for the boy's raging temperature.
The boy was coated in ice and thankfully he made a rapid and complete recovery. This was the first documented case of malignant hyperthermia, and the presentation is fairly classical, with all the tell-tale signs that we'll get into in this post.
What's supposed to happen
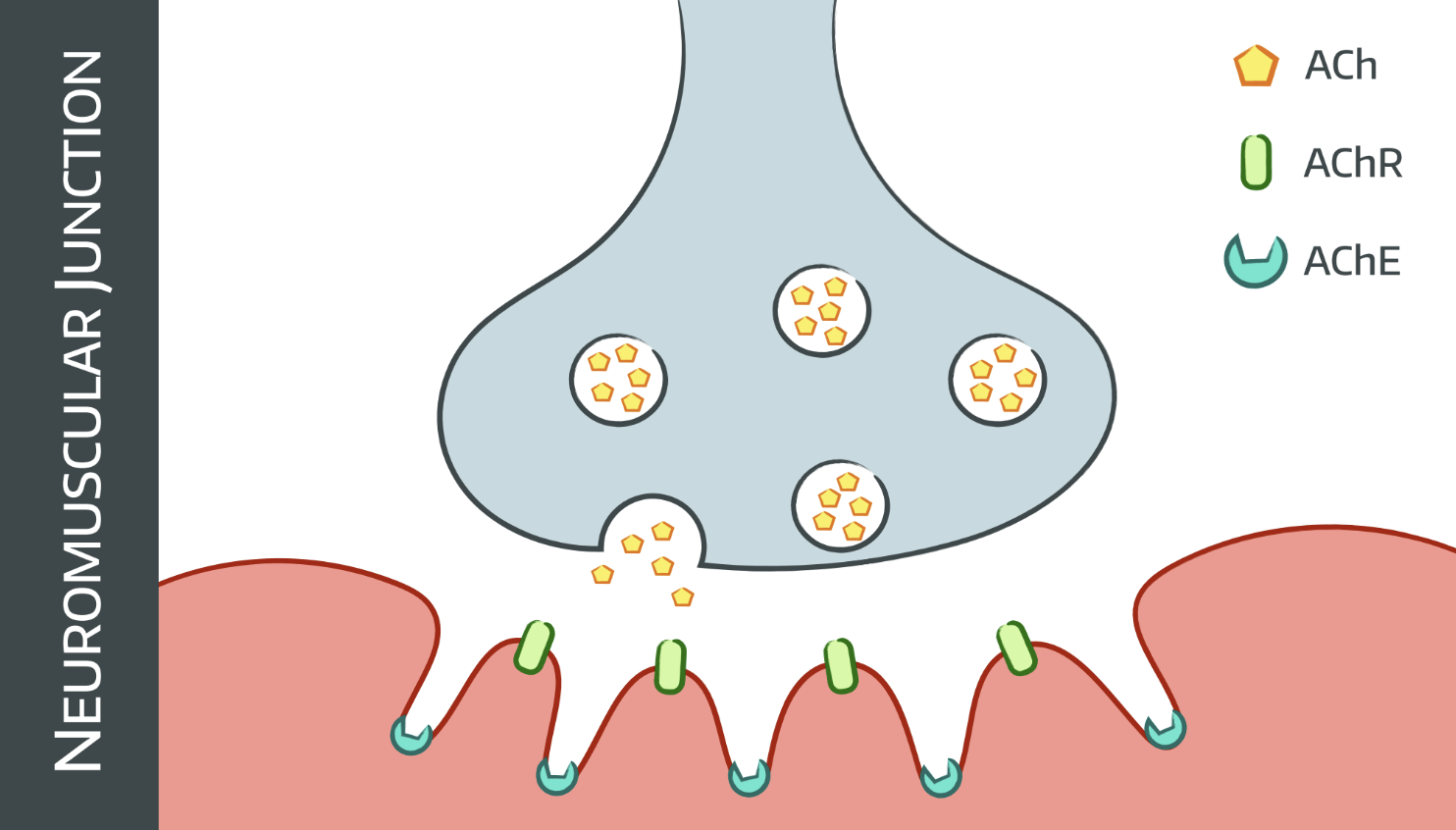
Here's a quick recap of the chain of events that we like to see in skeletal muscle:
- Action potential arrives at nerve terminal
- ACh release into neuromuscular junction
- Triggers AChR at motor end plate
- Cations enter ion channel
- Cell membrane depolarises
- Action potential spreads to muscle via T tubules system
- DHPR conformational change
- Ryanodine receptor triggers
- Sarcoplasmic reticulum releases calcium into cell
- Excitation-contraction coupling
- Muscle contraction
The channel that releases calcium from the sarcoplasmic reticulum is also called the ryanodine receptor (RYR1), as the plant alkaloid by the same name was originally used to identify it.
There's another receptor called the dihydropyridine receptor, which coordinates excitation-contraction coupling and has been found to also have the occasional mutation that can lead to MH, but the exam answer is the RYR1 ryanodine receptor.
What goes wrong
In Malignant Hyperthermia, there's a genetic structural problem of the ryanodine receptor.
This means that the above chain of events proceeds as normal right up until the ryanodine receptor starts allowing calcium to flow into the sarcoplasm from the sarcoplasmic reticulum.
However in MH this calcium release is completely uncontrolled, leading to rapid and continuous muscle contraction.
This clearly uses up an enormous amount of energy and oxygen, and produces vast quantities of CO2, heat and lactate, which is what we see in the clinical presentation.
How often it goes wrong
MH reactions vary from approximately 1 in 40,000 to 1 in 100,000 anaesthetics.
Genetic susceptibility varies between 1:5000 and 1:10,000 in the population.
It is autosomal dominant, so runs in families - meaning that pre-emptive testing and thorough history taking can save you a lot of stress.
It is thought that a susceptible person will react to suxamethonium on the first exposure, but may require repeated exposure to volatile agents before demonstrating a reaction.
What does it look like?
The problem is severe and sustained muscle contraction with no off switch. So thinking this through logically, it's going to have multiple effects that make an appearance on the monitor:
- Huge increase in oxygen demand (hypoxia and tachycardia)
- Huge increase in CO2 production (rising end tidal CO2)
- CO2 and lactic acid production (combined respiratory and metabolic acidosis)
- Rhabdomyolysis leads to hyperkalaemia (arrhythmias) and myoglobinaemia (renal tubular destruction)
- Muscle necrosis combined with rising core temperature can lead to disseminated intravascular coagulation (DIC)
The patient's core temperature can rise as fast as > 1°C per 10 min, but this presents far later than the increasing CO2 and tachycardia, so hopefully you've already started managing the situation before this becomes apparent.
A note on suxamethonium
If you use suxamethonium at induction, you know it's time to pick up the laryngoscope when the fasciculations stop, because it's a depolarising neuromuscular blocking agent.
A bit of muscle rigidity and masseter spasm upon administering suxamethonium is generally considered normal, and muscle aches and pains are moderately common after suxamethonium usage (can be avoided with a touch of rocuronium or atracurium - or by avoiding sux altogether).
However MH-susceptible patients have a much greater degree of masseter spasm - to the point whether the anaesthetist may be unable to open patient's mouth two minutes after administering suxamethonium.
This is a big red flag and warrants formal investigation for malignant hyperthermia.
What else could it be?
Malignant hyperthermia is a bit like anaphylaxis and pulmonary embolism, in that it ends up being a condition that you have to:
- Proactively consider in the first place
- Quickly rule out
As with many conditions in medicine, MH has several 'mimics' that look a lot like it, largely because of the relatively non-specific way that it presents itself - with an unexplained tachycardia, rising end tidal CO2 and evidence of metabolic overload and hypoxia.
What is the differential diagnosis for malignant hyperthermia?
- Inadequate depth of anaesthesia
- Anaphylaxis
- Sepsis
- Thyrotoxicosis
- Ischaemia
- Phaeochromocytoma
- Myopathy
How do I fix it?
Pull the buzzer and ask someone to grab the MH kit/trolley/bag if your department has one (if not - there's a free QIP for you)
- Stop the vapour
- 100% oxygen and hyperventilate
- Grab a new breathing circuit and stick the charcoal filters from the MH kit on
- Keep asleep with propofol
- Delegate someone to start drawing up dantrolene
- Start cooling the patient down - cool IV fluid, irrigation of body cavities, send someone to get ice, remove blankets and drapes (dialysis and bypass can be used as well if immediately available)
What does dantrolene do?
- Blocks the interface between the t-tubule system and the sarcoplasmic reticulum
- Prevents calcium-induced calcium release
There are 20mg dantrolene per bottle, with 3g mannitol to make it soluble.
Draw it up, and start giving it until there is improvement in heart rate, CO2 and temperature. This may need up to 10mg/kg but generally around 3mg/kg ends up being enough.
You might need to give more over the next 24-48 hours so keep it handy.
What other management is sensible?
- Insulin and dextrose for hyperkalaemia
- Bicarbonate for severe acidosis (contentious as always)
- Spot and manage coagulopathy as normal
- Spot and manage arrhythmias as normal
- Arterial line for blood pressure measurement and repeated arterial blood gas analysis
- Catheter
As you might expect after giving litres of cold fluid and many grams of mannitol - there's likely to be a profound diuresis headed your way - aim for 2ml per kilogram per hour at least to reduce kidney injury.
Who should I tell?
Well initially, lots of people, because you're going to need a load of hands to help institute the management.
Once you've (hopefully) treated the issue and taken the patient to intensive care, you should make a formal referral to the Leeds MH investigation unit, which is the UK's national MH centre.
Approximately 100–200 new cases are referred every year, and about 20-25% are found to be genuine MH reactions.
If a patient has been unfortunate enough to suffer from an episode of suspected malignant hyperthermia, then after the immediate and acute phases of resuscitation and recovery, they need to be thoroughly councelled and referred to the MH centre in Leeds.
They will want:
- A detailed anaesthetic chart with a thorough description of the event
- All bloods and investigations that were taken prior to the operation
- Plasma creatine kinase concentration 24 hours after the operation
How do they confirm the diagnosis?
- In vitro contracture testing (IVCT)
The patient undergoes a muscle biopsy (presumably not under the same general anaesthetic they just had) and the muscle strips are tested with halothane and caffeine.
If the muscle twitches signficantly in response to either or both, then they are considered to be MH-susceptible.
Podcast Episode
A free CRQ from FRCA-revision

Useful Tweets and Resources
The Association of Anaesthetists have a very helpful guideline along with these two pdf laminates that are freely available on their site, or you can grab them here:
(Technically they're archive documents but they contain useful info)
Our latest understanding of calcium dynamics in MH sk muscle using the p.G2435R model. Collaboration with colleagues in Australia @BradleyLauniko1. Some of the work done in the Leeds MH unit, @ukmhr @profhop funded by @The_MRC @BJAJournals and others.https://t.co/b4VRCXDNTx
— Vikas Kaura (@vikaskaura1) October 27, 2021
How to prepare your anaesthesia workstation for an MH- susceptible patient? @BillersJ @ukmhr @EMHG_EMHG @profhop#BJAEducation #anaesthesia #malignanthyperthermiahttps://t.co/uj8qoEjohY pic.twitter.com/VhTD82IG50
— British Journal of Anaesthesia (@BJAJournals) June 10, 2021
🔐Brand new for 2021 is this new #FOAMed @Assoc_Anaes malignant hyperthermia guideline from @profhop @ukmhr!
— 𝘈𝘯𝘢𝘦𝘴𝘵𝘩𝘦𝘴𝘪𝘢 (@Anaes_Journal) January 5, 2021
They are the first such guidelines to be published in a peer reviewed journal and replace the 2011 crisis management laminates.
🔗https://t.co/cXZdT1XBNH pic.twitter.com/cklGbVC5g6
The proportion of patients referred to malignant #hyperthermia units without a personal or family history of an adverse #anesthetic event has increased, with 47 of 120 diagnosed as malignant hyperthermia–susceptible. Read more: https://t.co/a5fynS3vUJ pic.twitter.com/D3jKPd4myB
— Anesthesiology (@_Anesthesiology) June 17, 2023
References and Further Reading


Primary FRCA Toolkit
Members receive 60% discount off the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.
