Isomerism

Take home messages
- Molecules can be arranged in a variety of different configurations, which can result in wildly different chemical properties despite having the same ingredients
- An understanding of this principle can help guide you to choose the best agent for your patient
What is isomerism?
In the same way that you can use the same combination of Lego blocks to build a car or a plane, isomers are molecules with the same chemical formula, but a different arrangement of atoms in space.
This can either form two different molecules with fairly similar properties:
- isoflurane and enflurane
or two combinations with very different properties:
- prednisolone and aldosterone
- dihydrocodeine and dobutamine
There are two broad categories of isomers
These can be structural isomers or stereoisomers.
Structural isomers
- These are molecules with the same chemical formula, but different structural arrangement of atoms throughout the molecule
There are two types of structural isomers:
- Static
- Dynamic
Stereoisomers
- These molecules have the same structural formula, and the same atoms are bonded to each other
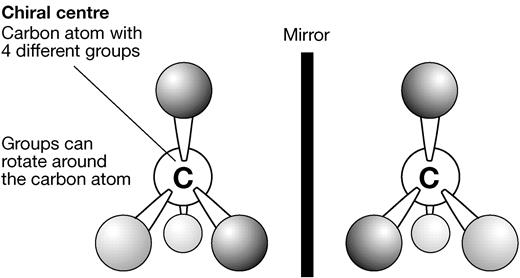
- However the molecule contains a tetravalent chiral centre (a molecule able to form four bonds, each bond connected to a different atom or group)
- This means there are different ways those four groups can be arranged around that chiral centre atom, producing configurations that can't be exactly superimposed on each other
A bit more about structural isomers
As mentioned above, structural isomers can be static or dynamic.
Static structural isomers
Static structural isomers permanently have a different arrangement of atoms in space, and there are three ways this can present - positional, chain or functional.
- Positional static structural isomers
Positional isomers have atoms occupying different positions on an identical carbon chain.
Examples would be isoflurane and enflurane
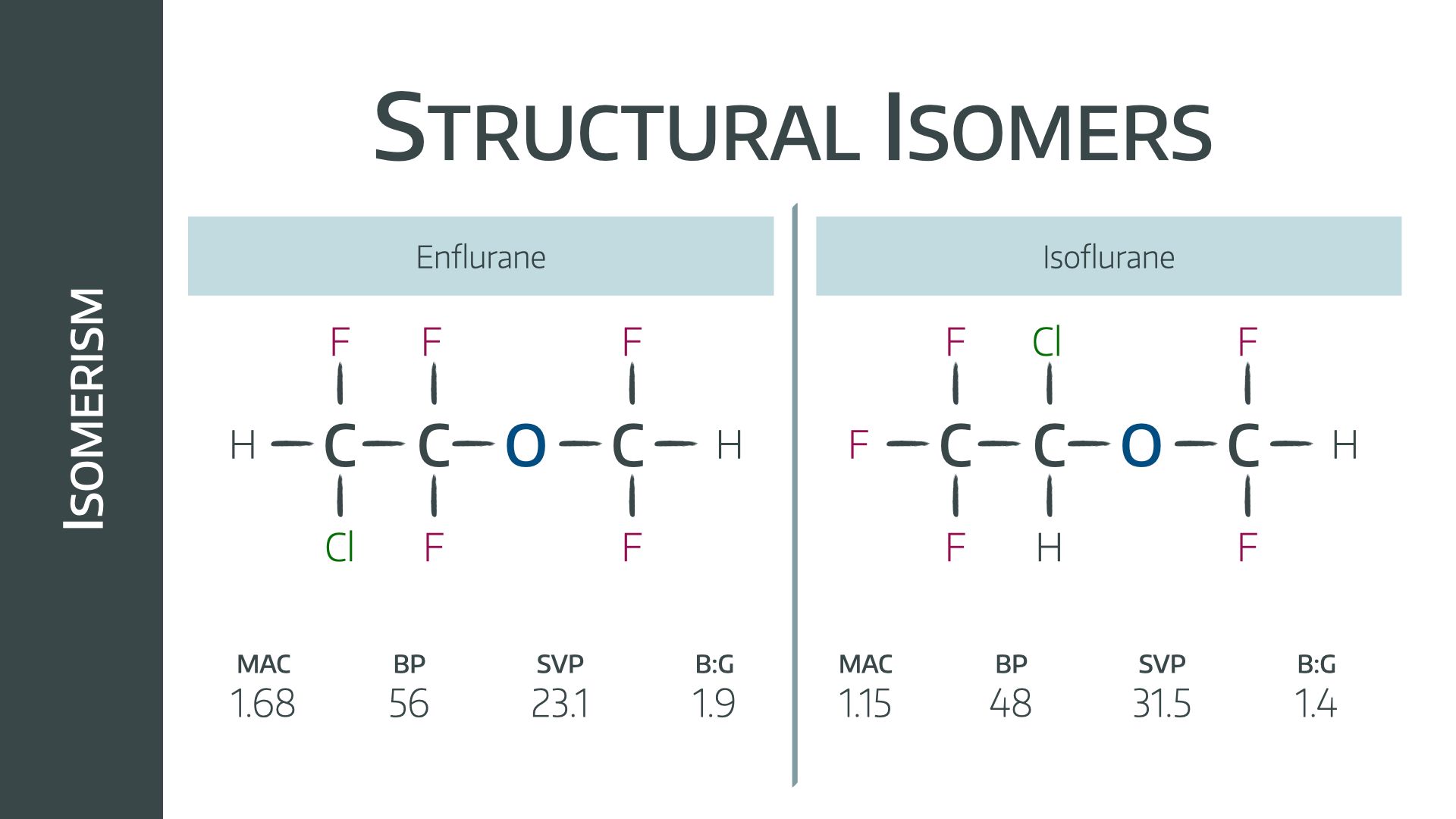
Both have remarkably similar properties, in particular a very similar oil:gas partition coefficient of 99 and 98 respectively.
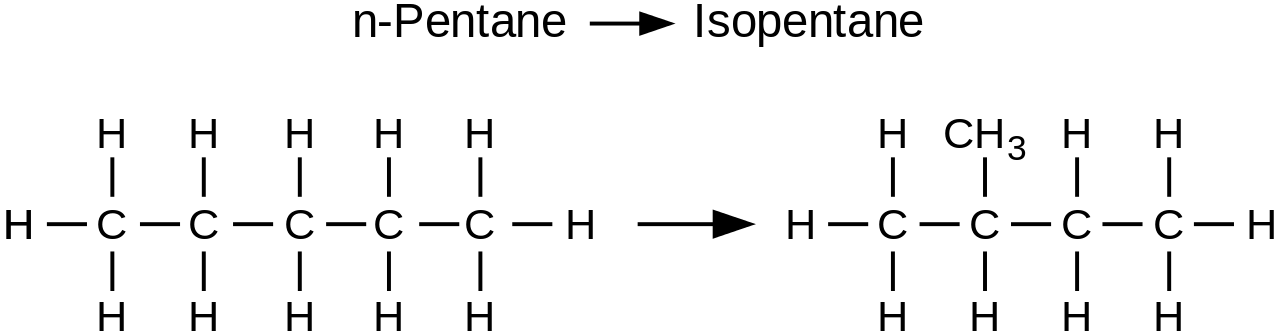
- Chain static structural isomers
Chain isomers have different carbon skeletons, such as pentane and isopentane, but the same functional groups.
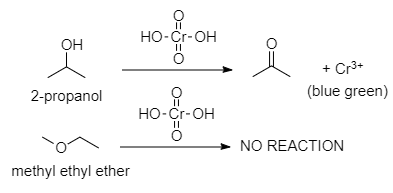
- Functional static structural isomers
Functional group isomers have different functional groups, such as methyl-ethyl ether and propranol. Propanol has an alcohol functional group, whereas methyl ethyl ether does not, and this gives it profoundly different chemical properties.
Dynamic structural isomers
These molecules undergo structural change depending on the environment in which they are placed, producing two different molecular arrangements with differing physicochemical properties.
The two commonly cited examples in anaesthesia are tautomerism and pH dependent ring closure
- Tautomerism (of thiopental)
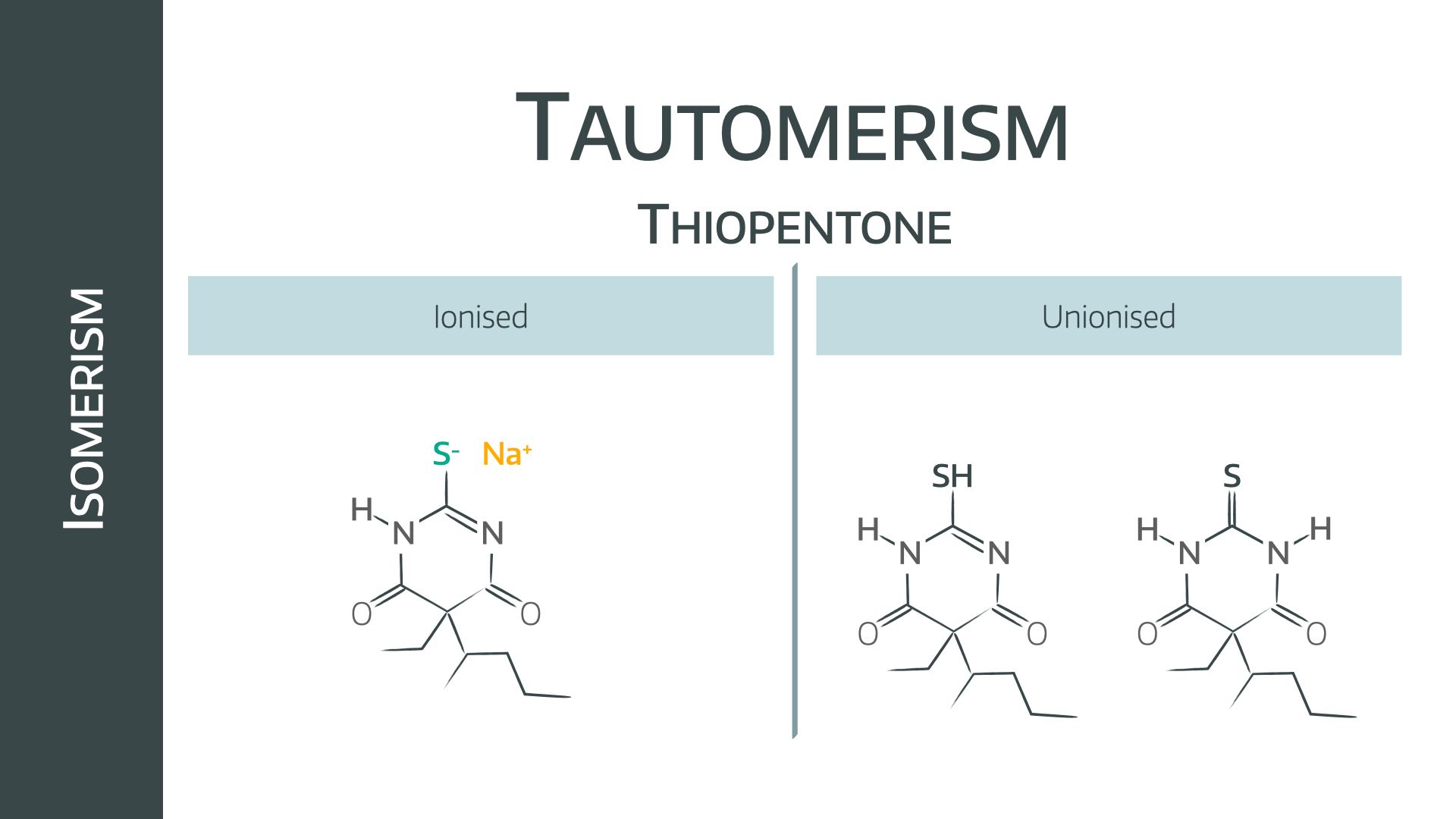
Soluble, ionised thiopental exists in an alkaline pH, hence the vial has added hydroxide to maintain solubility.
On injection into the blood, the pH drop causes the molecule to unionise into the thiol group, and then to rapidly convert to the pharmacologically active thiopentone as seen above.
- pH dependent ring closure (of midazolam)
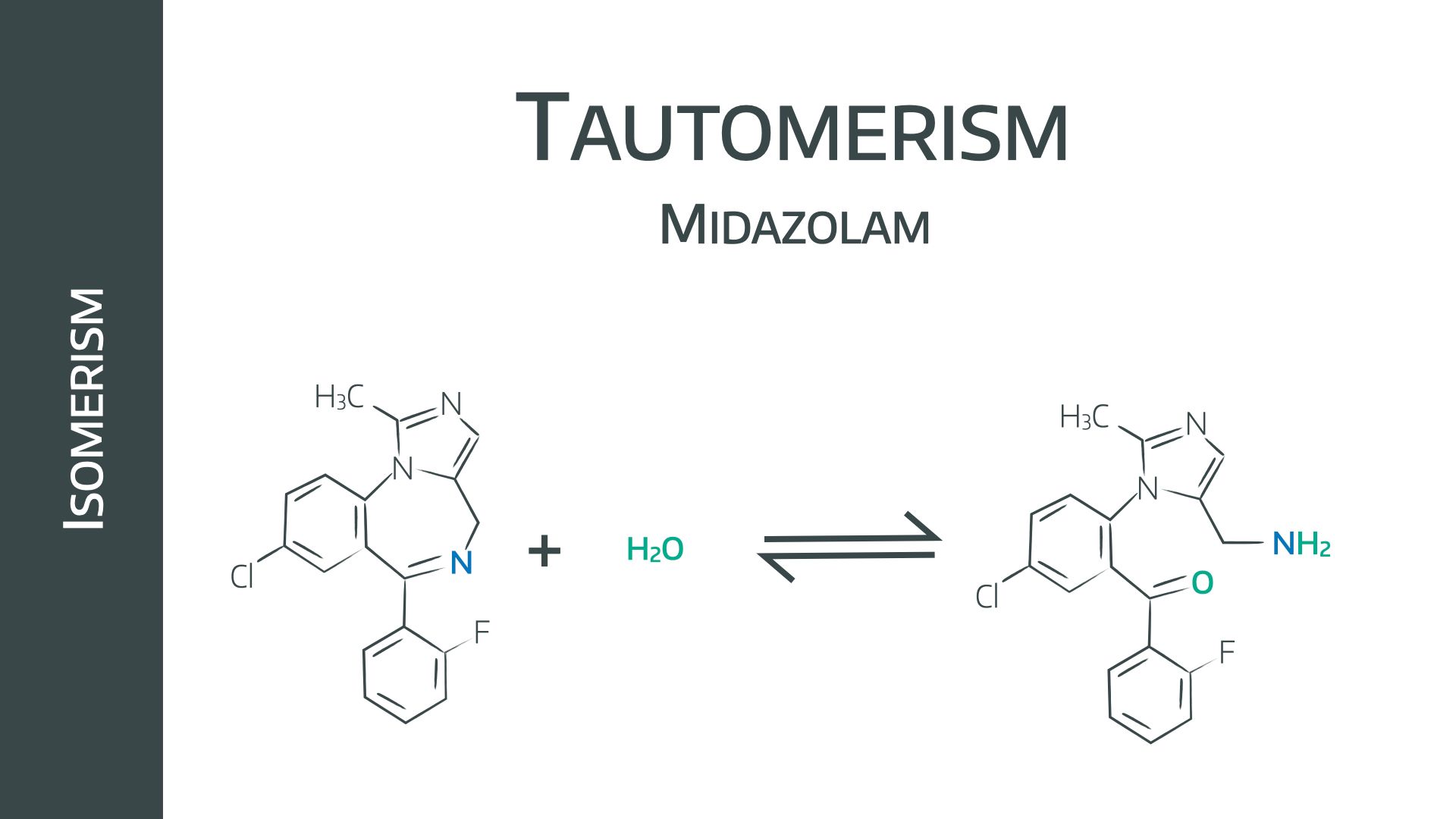
Midazolam contains a primary amine group which does the opposite to thiopental and is ionised in low pH environments and therefore soluble in water.
After being injected into the blood, pH-dependant ring closure occurs, forming a benzodiazepine ring. The now unionised molecule is lipid soluble and able to cross the blood brain barrier to exert its central effects.
A Quick Recap
- Isomers can be structural or stereoisomers
- Structural isomers can be static or dynamic
Static isomers can be:
- Positional (enflurane and isoflurane)
- Chain (pentane and isopentane)
- Functional (2-propanol and methyl ethyl ether)
Dynamic isomers are:
- Thiopental - tautomerism
- Midazolam - pH dependent ring closure
Now on to Stereoisomers
These molecules have the same chemical formula, and same broad structure, however the atoms have a different arrangement in space.
They are classed as either:
- geometric (cis-trans)
- optical (enantiomers).
Geometric stereoisomers
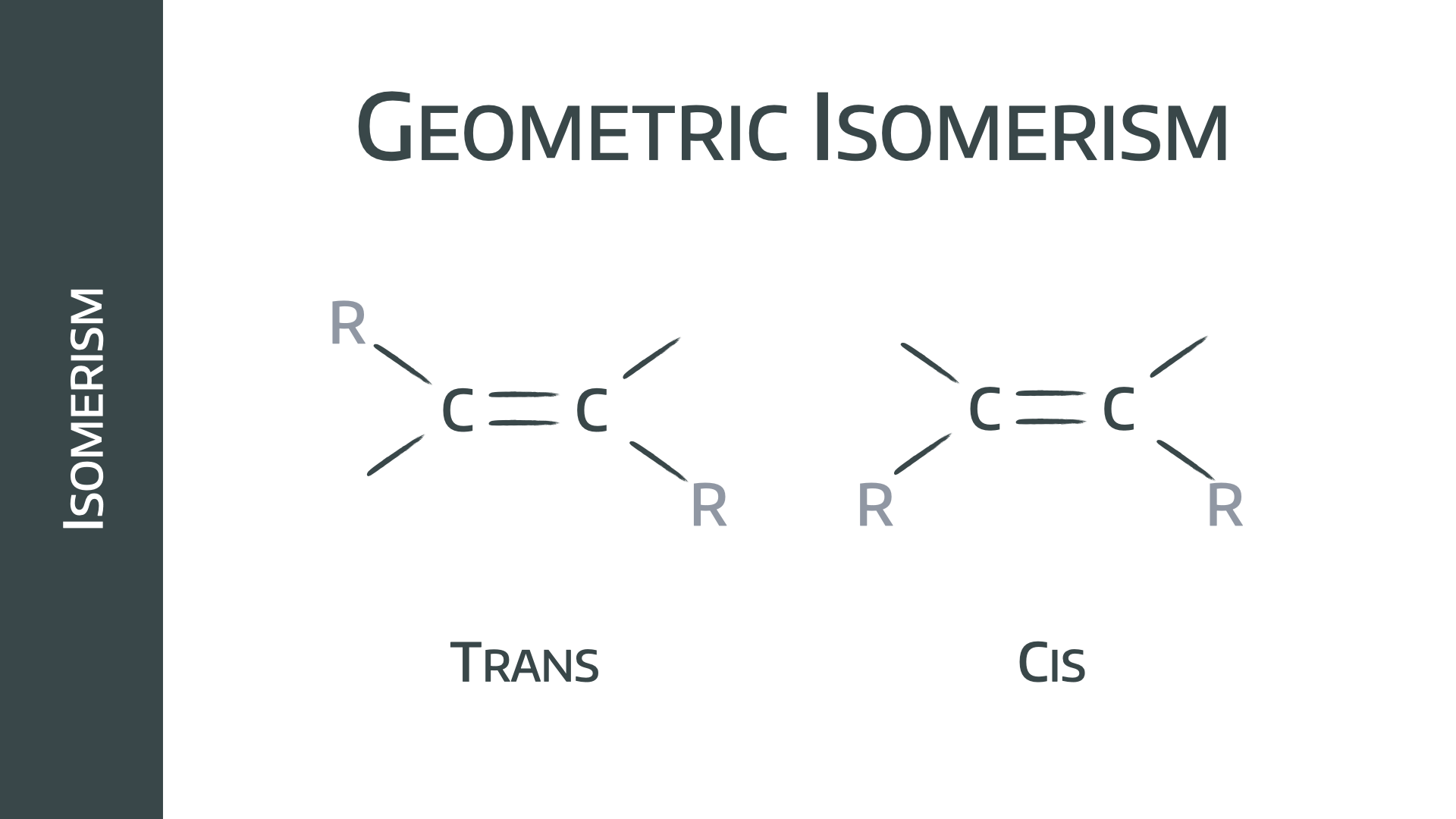
Geometric isomers rely on a carbon=carbon double bond, or aromatic ring, which is unable to rotate freely. The differing groups can then be arranged either diagonally opposing or on the same side of the bond, resulting in a different conformation and different chemical behaviour
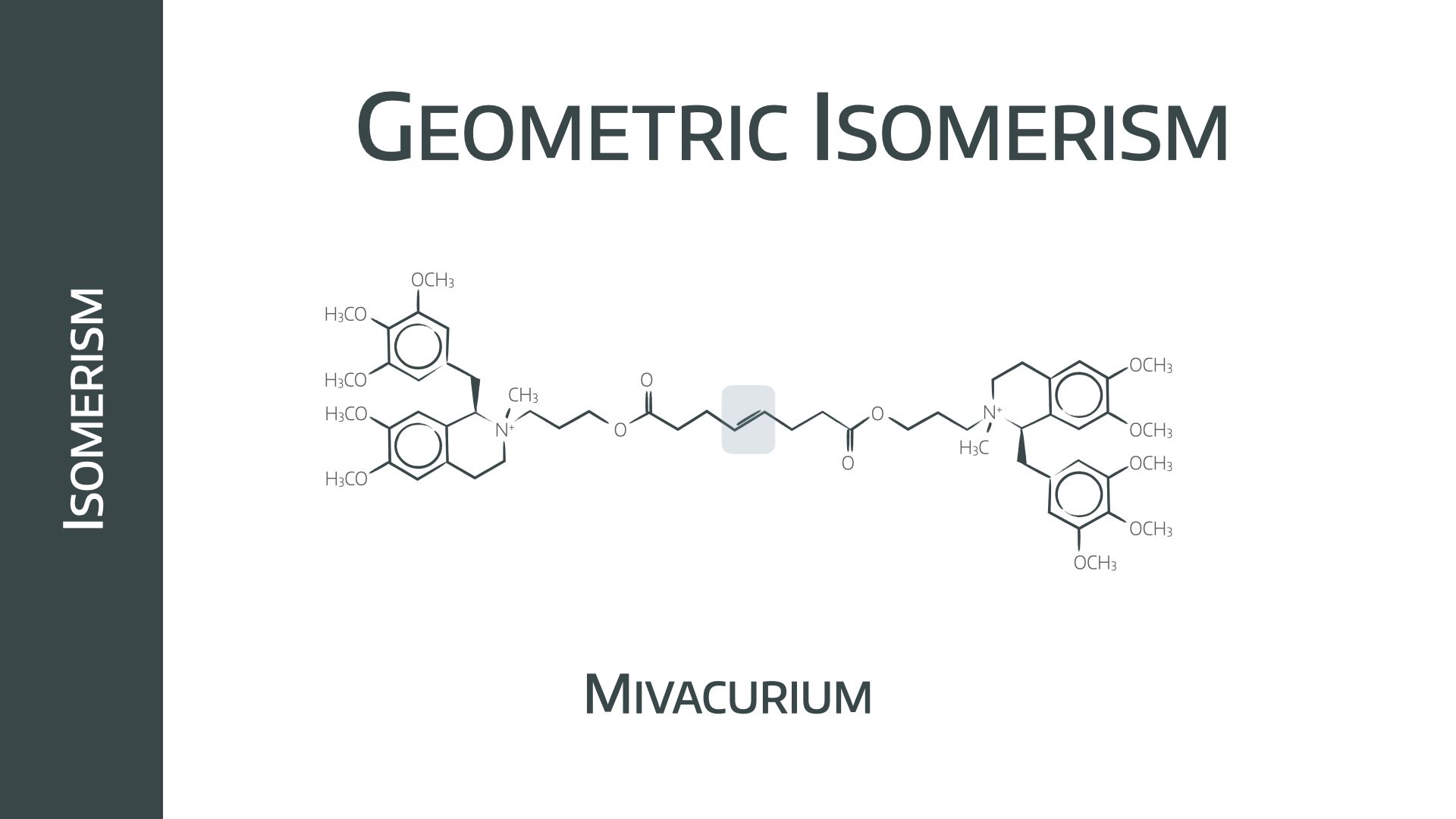
Mivacurium exists as three possible geometric isomers
- Trans-trans 58%
- Cis-trans 36%
- Cis-cis 6%
Optical stereoisomers
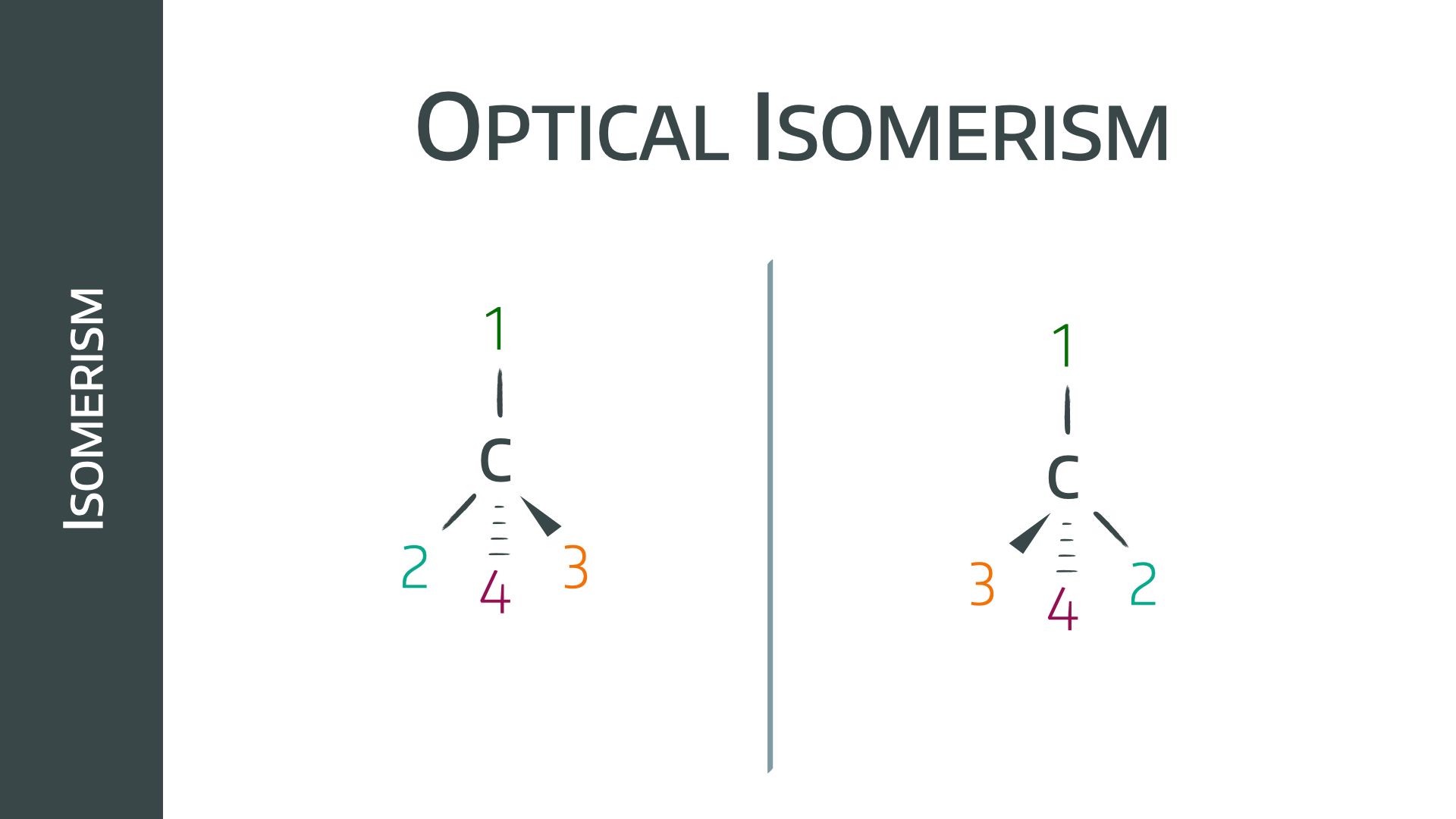
These molecules have a tetravalent chiral centre; a carbon (or nitrogen) molecule bound to four different groups.
It is possible to arrange these groups differently around the carbon centre in two different molecules, in such away that no amount of rotation will allow one to superimpose perfectly onto the other
Previously these were categorised based on their ability to deflect plane polarised light
- The enantiomer that deflected light to the left was the levo- enantiomer, such as levobupivacaine
- The enantiomer that deflected light to the right was the dextro- enantiomer, such as dextrobupivacaine
Dextrobupivacaine is significantly more cardiotoxic and neurotoxic than levobupivacaine, and is responsible for the majority of the toxicity caused by the racemic bupivacaine. For this reason levobupivacaine is often preferred as it is 'safer' from this point of view.
The modern classification uses the concepts of R and S instead
The description by Blandford explains it nicely:
"Imagining the atom with the lowest atomic number lies behind the plane of the page, the other three atoms will lie in the plane of the page. In the 'R' enantiomer, the atomic numbers descend in a clockwise manner. If the atomic numbers descend in an anti-clockwise manner, the enantiomer is the 'S' form” - Blandford
- The R+ isomer of etomidate is 10x more potent than the S− isomer in its action at the GABAa receptor
What are diastereoisomers?
Because the above system isn't quite complex enough, let's add another concept into the mix.
Diastereoisomers.
Some molecules have more than one chiral centre.
The multiple resulting configurations are not all mirror images of each other, and they cannot be superimposed either, so they are referred to as diastereoisomers because they will have slightly (or substantially) different physicochemical properties.
- A molecule with X number of chiral centres will have X^2 possible diastereoisomers
- However due to internal symmetry within the molecule, some of these may end up being duplicates
- This is why atracurium has 4 chiral centres but only 10 different formations
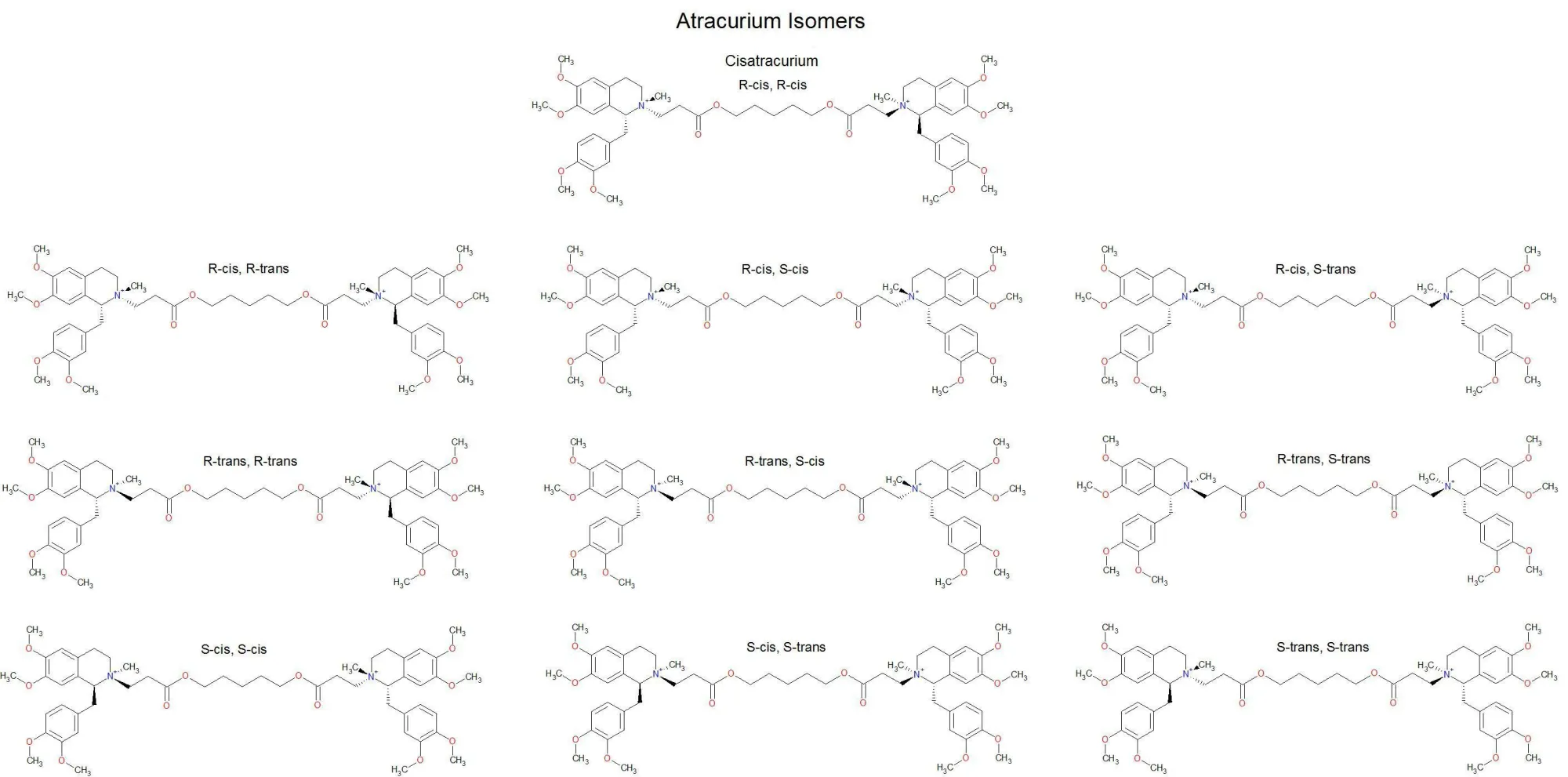
What is a racemic mixture and can you name any?
A mixture of isomers in equal proportion
- Isoflurane
- Ketamine - can also be produced as an enantiopure solution containing esketamine (literally S-ketamine)
- Adrenaline
- Warfarin
Interestingly, molecules produced naturally tend to exist as single isomers, as the enzyme that produces them is only capable of producing that conformation of atoms.
It is generally when molecules are synthesised artificially that we see racemic mixtures occurring as the purification processes often cause racemisation.
FRCA Primary Syllabus
- PR_BK_04 Isomers: structural and stereoisomers: classification systems; clinical relevance
Useful Tweets and Resources
If you haven't yet discovered this fabulous and free question bank, be sure to check it out!
References and Further Reading
Primary FRCA Toolkit
Members receive 60% discount off the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.

