Cerebral Blood Flow and Intracranial Pressure
Take home messages
- Intracranial pressure is often examined, and an easy place to collect lots of extra marks
- Understanding the factors that influence intracranial pressure is crucial to managing patients with head trauma effectively
- In district general hospitals where intracranial pressure monitoring isn't available, you may have to 'assume' a raised pressure to be on the safe side
Podcast episode
A spot of history
A long long time ago, before anaesthesia and CT scans - or medicine really - if you behaved strangely it was 100% because there was a demon in your head and the best way to release it was with a drill to the skull.
Preferably while wearing an upside down funnel, and with your ODP balancing the controlled drugs book on their head.

This procedure dates back as far as 10,000 years ago, and amazingly, the mortality rate (for an unsterile, unguided drill to the skull in a field) is believed to only have been around 60%.
While the techniques and indications have evolved and improved somewhat, the underlying principle for a lot of intracranial pathology is the same:
The skull is a closed box
Enter the Monro-Kellie doctrine
The skull is a fixed container with a lot of little holes, and one big one at the bottom. Inside said fixed box of bone there exist three types of content:
- Brain 85%
- Blood 5%
- CSF 10%
They live in a constant harmonious balance wherein if any one of them increases in volume, to avoid a harmful rise in intracranial pressure, one or both of the others must decrease in volume to compensate.
Otherwise you get this graph:
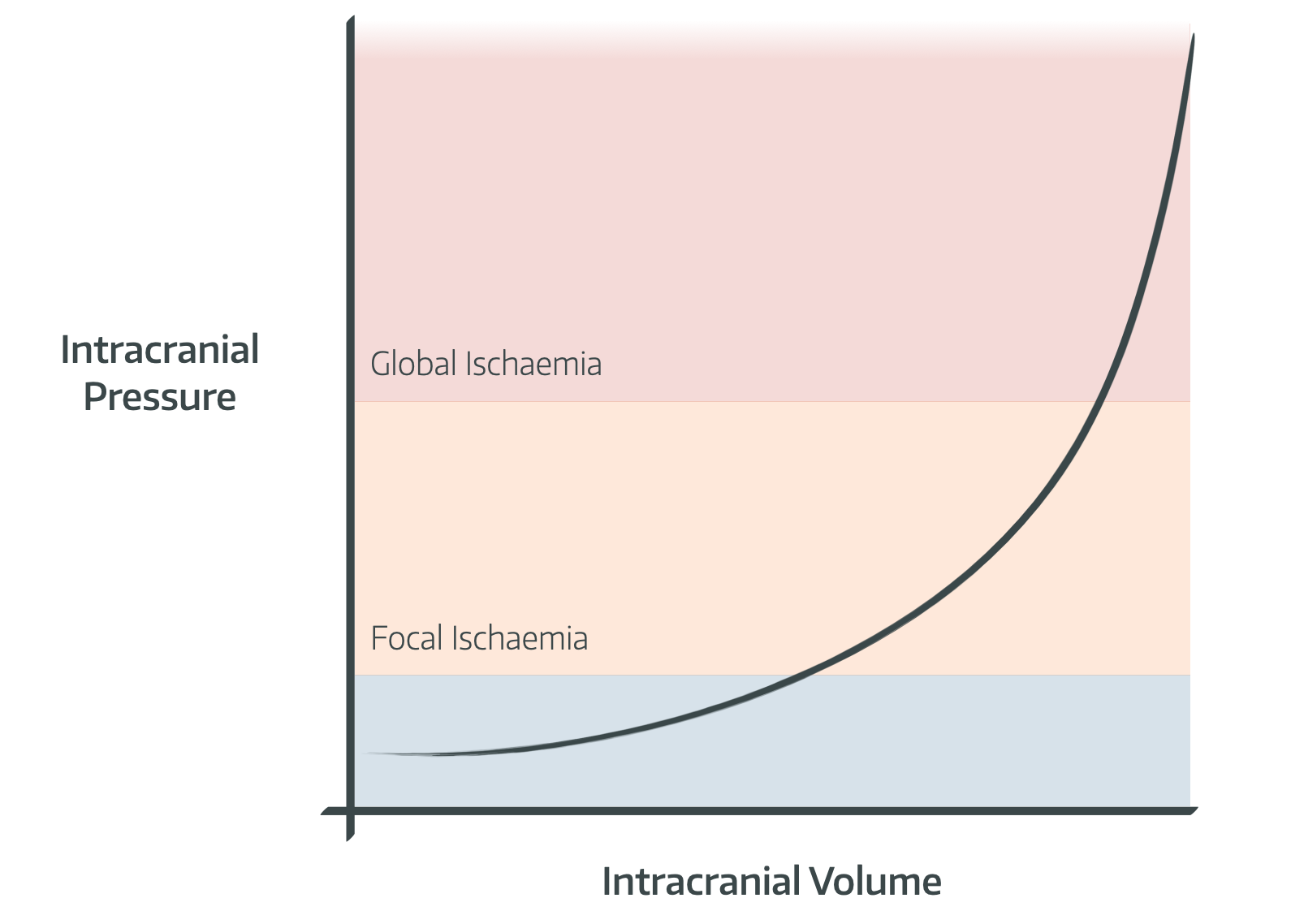
- Note that normal pressure, when lying supine, is approximately 8-12mmHg
- Above 20mmHg there starts to be focal ischaemia, and above 45-50mmHg there is global ischaemia
What are the causes of raised intracranial pressure?
This is a very common exam question, either in the SOE or the OSCE. The key to a strong answer is, as always, to categorise.
Increased CSF in the brain:
- Overproduction
- Underabsorption
- Blockage of drainage
- All three of these can lead to Hydrocephalus
Mass effect of the brain itself:
- Oedema
- Contusion
- Abscess
- Tumour
Increased intracranial blood volume:
- Venous obstruction
- Venous Sinus Thrombosis
- Haemorrhage
Benign intracranial hypertension:
- Unclear cause
- Associated with obesity
What happens when intracranial pressure starts to increase?
There are three main compensatory mechanisms in place to deal with increasing intracranial pressure.
- Initially venous blood is pushed out of the skull, reducing the volume of venous blood in the great veins and sinuses
This is why it's a good idea to avoid jugular central lines, and anything else that might obstruct venous drainage such as tube ties (use tape instead).
- Secondly, cerebrospinal fluid is extruded via the foramen magnum
There's a total volume of approximately 150ml of csf, with around 125ml of this in the subarachnoid space (both brain and spinal cord), and only about 25ml in the ventricles.
- Thirdly the volume of arterial blood starts to reduce
This is where it becomes seriously bad news, because the blood supply to an already stressed brain decreases.
After a certain point, there won't be anything else that can be removed to accomodate the expanding volume, and only very small increases in volume will dramatically increase intracranial pressure, causing compression of the brain parenchyma and ischaemia.
What are the clinical features of raised intracranial pressure?
- Headache, worse on straining or bending over
- Nausea and vomiting
- Papilloedema
- Reduced GCS or irritability
- Bulging fontanelle in infants
The Cushing Reflex is a preterminal sign
- Raised intracranial pressure leads to hypoperfusion
- The brain releases vasoactive mediators to increase blood pressure
- This increased blood pressure causes a reflex bradycardia, and further increases intracranial pressure
- The increasing pressure leads to herniation of brainstem through foramen magnum, stretching the cranial nerves
- This is known as coning
- Hypotension, apnoea and fixed dilated pupils follow once cushing’s reflex fails
Talk to me about cerebral blood flow
As one might expect, the brain receives rather a lot of blood in order to sustain all of those thoughts and decisions, and ends up receiving around 25% of the cardiac output.
The golden number to remember is 50 ml of oxygen per 100g of brain tissue per minute.
Factors influencing cerebral blood flow relate to the Hagen Poiseuille equation:
Pressure gradient
Cerebral Perfusion Pressure = Mean Arterial Pressure – (ICP + CVP)
- Where ICP = intracranial pressure and CVP = central venous pressure
- Also CPP = Cerebral blood flow x Cerebral vascular resistance
- This is essentially Ohm’s law (V=IR)
Blood vessel diameter
Again similar to Ohm's law, the blood vessel diameter dramatically affects the resistance to blood flow
The main factors that induce vasoconstriction and dilation are PaCO2 and PaO2
PaCO2

Cerebral blood flow increases linearly with PaCO2 as a result of vasodilatation, between 2kPa and 10kPa. The graph plateaus at either end as a result of maximal vasodilatation and vasoconstriction.
In chronic hypercapnoea the graph is shifted to the right as a result of compensatory buffering. Bicarbonate is actively pumped into the CSF to buffer the increased hydrogen ion concentration caused by chronic hypercapnoea.
PaO2

Cerebral blood flow is stable above a PaO2 of 8kPa. Below this, vasodilatation causes a rapid increase in cerebral blood flow. This response is very strong and will overpower any vasoconstriction caused by other mechanisms.
What is flow-metabolism coupling?
The rate at which the brain consumes oxygen for metabolism is the cerebral metabolic rate or oxygen utilisation or CMRO2
- Usually around 3ml/100g/minute
- 5ml/100g/minute in young children
Flow-metabolism coupling is where perfusion is adjusted to match the metabolic demand of a given region of the brain, or the brain as a whole
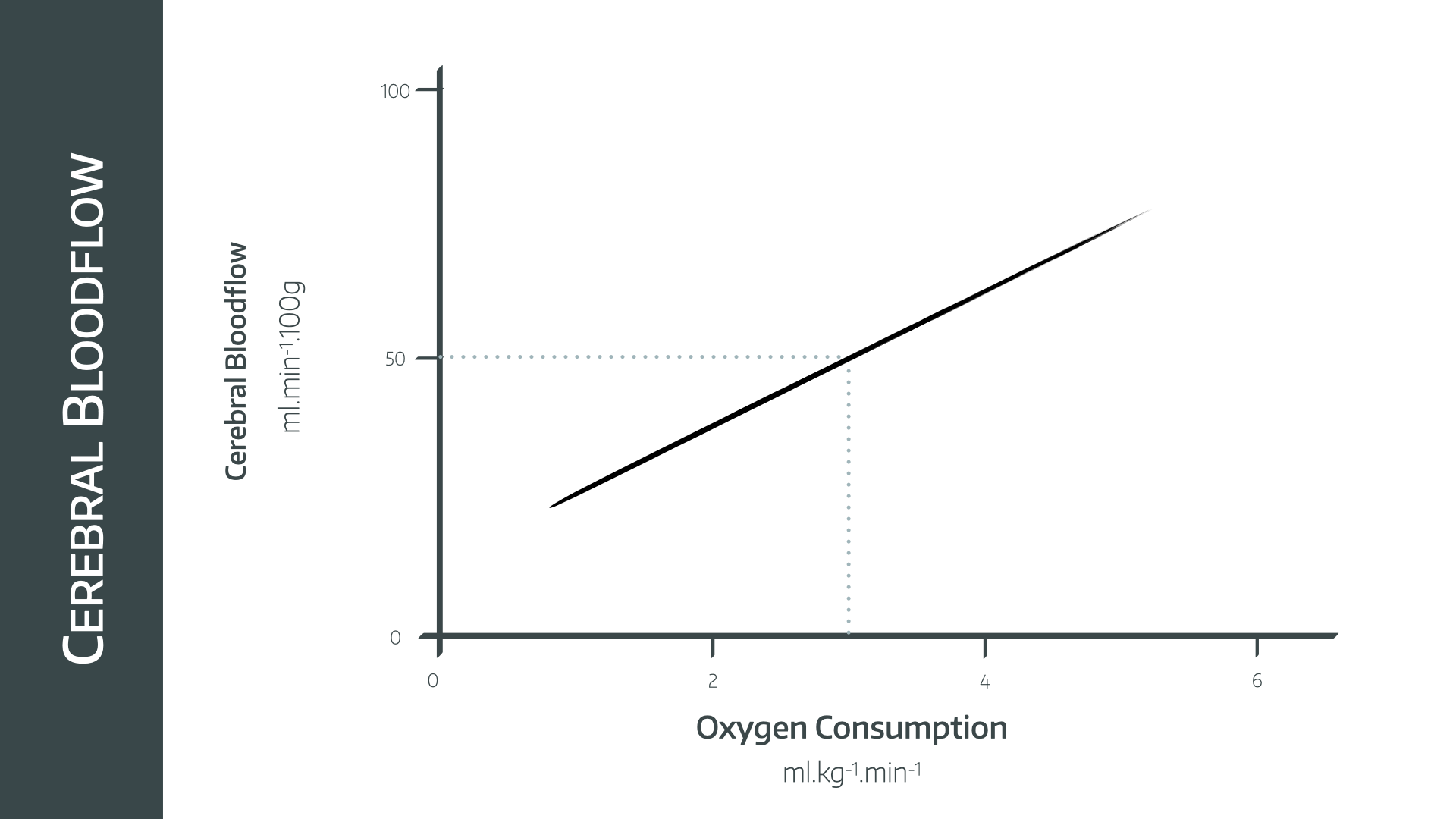
- There is a linear relationship between CBF and CMRO2
- Ischaemia occurs below 20ml/100g/minute
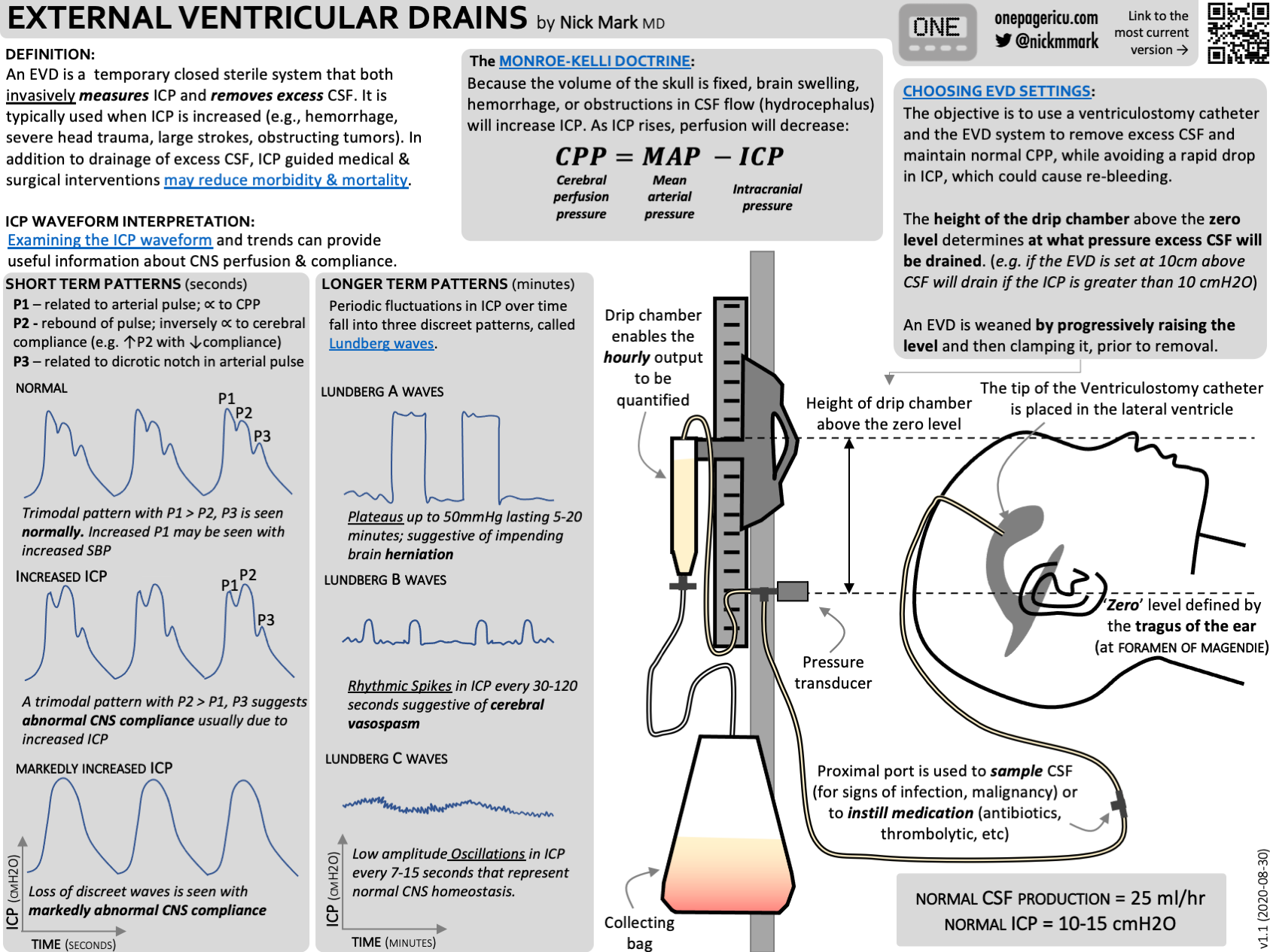
Can you draw and explain the intracranial pressure waveform?

This essentially looks a lot like the invasive arterial pressure waveform, measured through an intracranial pressure transducer such as a ventricular drain or subarachnoid bolt.
It has three pulse waves, each with gradually reducing amplitude.
Pulse wave
Pulse waves
P1
- Percussive
- Transmitted arterial pressure
P2
- Tidal
- Due to arterial wave reflecting off brain tissue, so varies with brain compliance
- Approximately 80% amplitude of P1
- Ends at point of dicrotic notch
P3
- Dicrotic wave
- Venous pressure wave, increases as CVP increases
As well as the three pulse waves in each beat, there are two other patterns in the pressure waveform:
Respiratory wave
This is a larger variation in baseline pressure with the respiratory cycle and is totally normal.
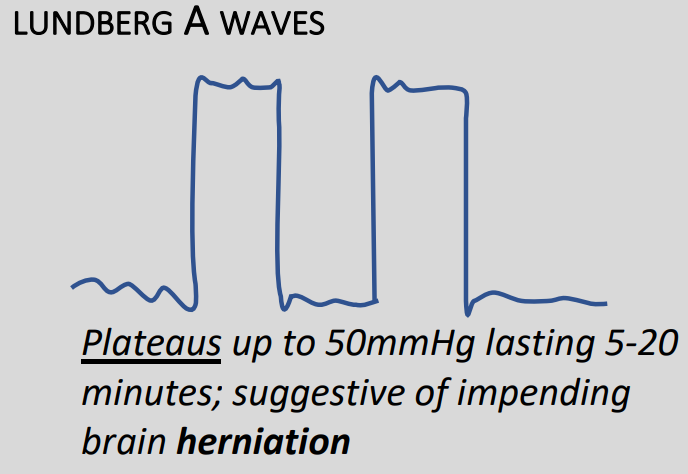
Slow waves
Also known as Lundberg waves, these are usually pathological, but not always.
Type A – Plateau waves
- Rapid increase of baseline pressure to more than 50mmHg for between 2 and 20 minutes
- Demonstrate severely reduce compliance
- Always pathological
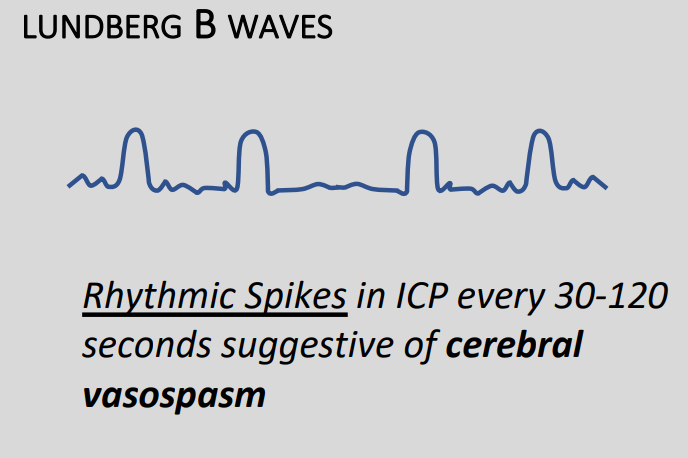
Type B
- 0.5 to 2 waves per minute
- Increases in pressure of around 20-30mmHg before returning to baseline
- Variable ICP usually present as well
- Sometimes due to vasospasm
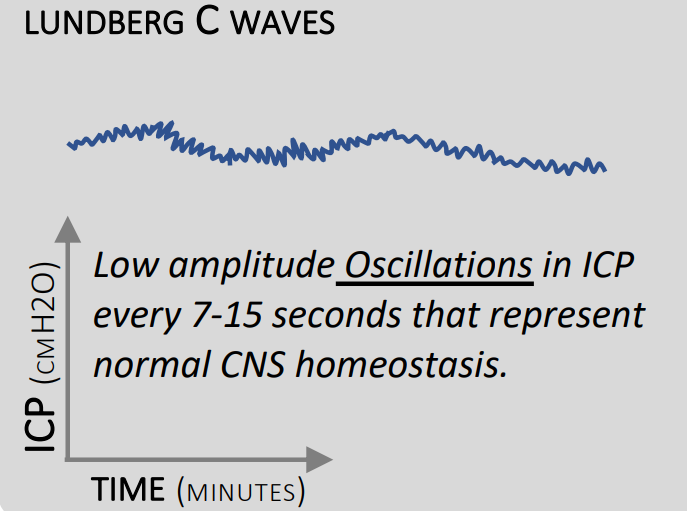
Type C
- 4 to 8 waves per minute
- Up to 20mmHg
- May be normal
These images are beautifully represented in Dr Nick Mark's fabulous ICU one pager infographic, which you can find here, or in the resources section below.
How does carbon dioxide affect intracranial pressure?
Hypercapnia causes vasodilatation and therefore increased cerebral blood flow as detailed above.
Across the normal range of PaCO2, this results in a near-linear increase in cerebral blood flow.
However above 11kPa, maximal vasodilatation is reached, and so there is minimal further increase in cerebral blood flow, and the graph levels out. At low PaCO2 this also occurs, as the vasoconstriction caused by hypocapnoea is opposed by vasodilation induced by the resulting ischaemia and hypoxia.
Low CO2 tries to vasoconstrict, causing hypoxia which overrides it and forces the vessels to vasodilate
Should we hyperventilate patients with high intracranial pressure?
This seems like a logical plan on the face of it - by hyperventilating our patient we could theoretically induce a degree of vasoconstriction that would reduce intracranial blood volume and therefore intracranial pressure.
However there is a problem with this:
- It's a very temporary effect, and the brain rapidly recalibrates its set point for PaCO2 and will vasodilate again within a matter of a few hours
- Then when the hyperventilation stops, and the CO2 rises, the resulting vasodilatation would be even worse than before
Therefore hyperventilation should only be done in extremis as a temporising measure, for example in the ambulance when you're fifteen minutes away from definitive surgery.
For all other scenarios, the focus should be on maintaining a low-normal PaCO2, and preventing avoidable vasodilatation.
Aim for PaCO2 of 4-4.5kPa
Autoregulation

Autoregulation is the process by which an organ regulates its own blood flow, independent of perfusion pressure (within limits). Both the brain and kidney demonstrate autoregulation.
Between 50 and 150mmHg of mean arterial pressure, the cerebral blood flow remains at 50ml/100g/min.
This graph shifts to the right in chronic hypertension, emphasising the importance of maintaining sufficient MAP in known hypertensive patients.
What effect does temperature have on CMRO2?

As temperature decreases CMRO2 decreases, which is part of the rationale behind targeted temperature management in out of hospital arrest patients. The graph has two linear segments, changing gradient at 27°C.
- 7% reduction for every 1°C temperature drop
- At 27°C it will be 30% of baseline metabolic rate
- At 17°C it will be 10% of baseline
What effect do anaesthetic agents have on CMRO2?

The dashed line is the graph depicting flow-metabolism coupling.
Inhalational agents
Inhalational agents induce a dose-dependent vasodilatation apart from sevoflurane. At 1.5 MAC cerebral autoregulation is disrupted. Nitrous causes vasodilatation and also increases CMRO2.
Intravenous agents
Propofol and Thiopentone reduce CMRO2 and cerebral blood flow. Ketamine increases CMRO2 and cerebral blood flow, but flow to a greater extent. It is generally avoided in patients with raised intracranial pressure however it is often used for head trauma patients where cardiovascular stability is the priority.
What are the management options for raised intracranial pressure?
This is a common question at a variety of points during the FRCA exam journey, so it's worth investing some time to build a slick answer.
The categories we would suggest are pharmacological, non-pharmacological and surgical.
Pharmacological
- Noradrenaline - to maintain perfusing pressure
- IV induction agents (propofol, thiopentone, etomidate) - cause a dose dependent decrease in cerebral blood flow, pressure and metabolic rate
- Benzodiazepines - reduce blood flow and metabolic rate but not pressure
- Opiates - can decrease CMRO2 in large doses
- Muscle relaxants - useful to prevent coughing and straining
Just note that suxamethonium causes a transient increase in ICP, attenuated by induction agents such as thiopentone.
- Volatile agents - slight theoretical increase in ICP due to cerebral vasodilatation, but also reduce CMRO2 - Can be used at MAC <1
- N2O and Ketamine - Vasodilates and increases CMRO2, increasing ICP
- Barbiturates - deep thiopentone coma used in refractory seizures
- Phenytoin - used to prevent convulsions, it is a membrane stabiliser that prevents sodium and calcium influx during depolarisation
- IV fluids:
Mannitol
- Osmotic diuretic, filtered and not reabsorbed
- Withdraws water from brain across BBB
- Free radical scavenger and reduces CSF production
- 0.5 – 1ml/kg of 20% solution
Hypertonic saline
- 2-3ml/kg of 5% NaCl
- Hypertonicity draws fluid out of the interstitium into the blood to be drained away
Non-pharmacological
Main priorities
- Maintain oxygenation
- Prevent secondary brain injury
- Decrease ICP
- Optimise CPP
Ventilation
- PaCO2 4.0-4.5
- Hypocapnoea should be avoided to prevent vasoconstriction
Aid venous drainage
- Posture
- 30 degrees head up
- Head in midline
- No collars or tube ties
- PEEP minimal
Cooling is controversial
- Not shown to improve mortality however avoiding pyrexia does improve outcome
Surgical
Immediate neurosurgery is the only definitive treatment for raised intracranial pressure
- Debulking
- Drainage - via external ventricular drain
- Craniectomy
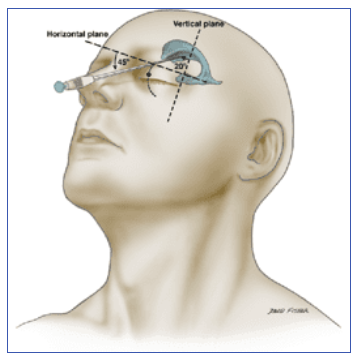
Apparently
You can put a needle through the orbit into the lateral ventricle and drain CSF that way...
Syllabus
- PB_BK_69 Intracranial pressure: cerebrospinal fluid, blood flow
A Free Final CRQ
Our wonderful colleagues over at FRCA-revision have put together a whole series of high yield CRQ questions for the Final FRCA, and they also have a fantastic SBA and MCQ bank for the Primary. Tap the image below to go straight to the CRQ on intracranial pressure.

Useful Tweets and Resources
If you haven't already discovered Dr Nick Mark's amazing infographics over at ICU one pager - you simply must check them out
Cerebrospinal fluid shooting out in acute hydrocephalus with elevated intracranial pressure pic.twitter.com/hzqCbRmgQk
— Oren Gottfried, MD (@OGdukeneurosurg) April 19, 2023
From our #neuroanaesthesia series, our #cognitiveaid on effectively managing elevated intracranial pressure. #cognitiveaids #ascar #anaesthesia #anesthesia #icu #anesthesiology #icu #icunurse #ernurse #erdoc #icudoctor #foamed #medicalstudent #medicalschool pic.twitter.com/13JVuPPGwz
— ascargroup (@ascargroup) January 11, 2023

Looking for an audit idea?
Check out the RCOA's audit recipe book for neuroanaesthesia below.

References and Further Reading


Primary FRCA Toolkit
Members receive 60% discount off the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Tap the image below to try our free demo toolkit

Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.

